Safety In Chickens
A comprehensive safety program was conducted to demonstrate the safety of Exzolt for chickens. One study investigated safety in laying hens, the primary production class for Exzolt, while another study evaluated safety in young 3-week-old chicks. A reproductive safety study was also conducted, evaluating safety in both treated breeder birds and their eggs/progeny. Outcomes obtained in these studies support the safety of Exzolt in pullets, breeders, and layers, per label indications.
- Two target animal safety studies demonstrated that Exzolt was well tolerated and normally palatable in both very young birds as well as adult hens under high physiological stress related to intensive egg production, even when dosed at 5-times the recommended dose for 3-times the recommended duration of treatment (no adverse impacts on health, egg production, or growth performance).
- A reproductive safety study demonstrated that Exzolt is well tolerated in breeder chickens, even at 6-times the intended total dosage (no adverse effects on fertility, hatchability, chick viability, or overall reproductive performance).
- Use of Exzolt at the recommended daily dose rate of 0.5 mg/kg BW twice at a 7-day interval clearly offers a wide margin of safety for all classes of pullets, breeders, and layers and has no impact on water consumption.
Study Design
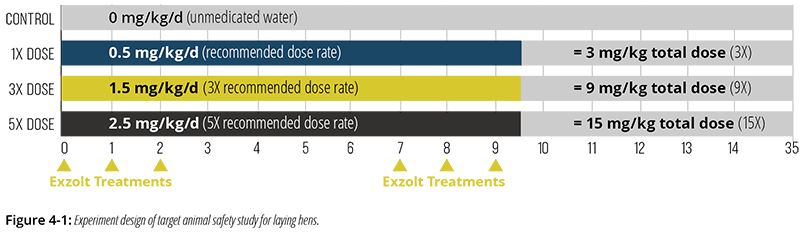
A pivotal study was designed to evaluate the safety of Exzolt treatment via drinking water when administered at 0 (control), 1-, 3-, or 5-times the recommended dose (0, 0.5, 1.5, or 2.5 mg fluralaner/kg BW, respectively) for 3-times the recommended frequency (6 treatments instead of 2), for a total overall dosage up to 15-times the recommended dose (Figure 4-1).1
- 120 laying hens at peak egg production (~28 weeks of age; under high physiological stress related to intensive egg production) were randomly allocated to 4 treatment groups of 30 birds.
- The 3 treated groups were offered tap water medicated with Exzolt as described in Figure 4-1 on 6 occasions (days 0, 1, 2, 7, 8, 9), while one control group received nomedicated water. (As a result, the total net doses of fluralaner administered were 3-, 9-, or 15-times the recommended total dose.)
- Clinical health was monitored throughout the 35-day study, and feed and water intake were recorded. Egg production was assessed daily for each treatment group, and eggs from 10 randomly pre-selected birds in each group were evaluated for typical quality parameters. Hematology and clinical chemistry parameters were measured pre-treatment and at multiple time-points.
- Eight birds per group were euthanized on days 10 and 28 for complete gross necropsy, and complete histopathological examination was performed on organs from the control and the highest dose (5-times) group.
- Data collections were performed by personnel blinded to treatment.
RESULTS
Evaluations of the massive quantity of collected data revealed no clinically significant or relevant differences for any safety or toxicological parameters between the control group and all medicated groups, including birds treated at the 5-times dose rate. Furthermore, no statistically significant differences between groups were detected for egg production, the number of abnormal eggs, or egg quality characteristics.
Water intake was similar between all treatment groups, indicating that normal palatability was maintained for drinking water medicated with Exzolt.
CONCLUSION
This target animal safety study demonstrated that Exzolt is well tolerated and normally palatable in laying hens, even when grossly overdosed at up to 5-times the recommended dose rate for 3-times the recommended frequency. Use of Exzolt at the recommended daily dose rate of 0.5 mg/kg BW twice at a 7-day interval offers a wide margin of safety for laying hens and does not impact rates of water consumption.
Study Design
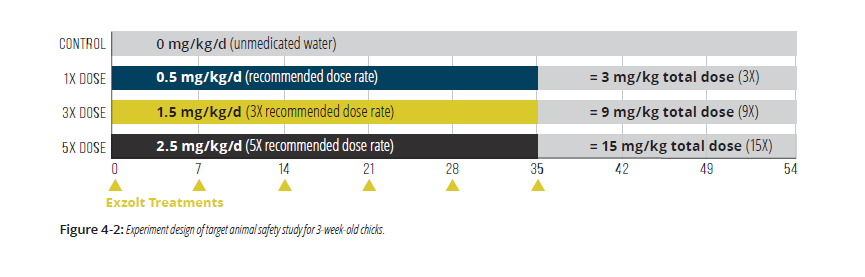
A similar pivotal study involving young chickens evaluated the safety Exzolt treatment via drinking water when administered at 0 (control), 1-, 3-, or 5-times the recommended dose (0, 0.5, 1.5, or 2.5 mg fluralaner/kg BW, respectively) for 3-times the recommended frequency (6 treatments instead of 2), for a total overall dosage up to 15-times the recommended dose (Figure 4-2).2
- 320 broiler male and female chicks (3 weeks of age at start of dosing) were randomly allocated to 4 treatment groups of 80 birds.
- The 3 treated groups were offered tap water medicated with Exzolt as described in Figure 4-2 on 6 occasions at weekly intervals (days 0, 7, 14, 21, 28, 35) while control group birds received non-medicated water. (As a result, the total net doses of fluralaner administered were 3-times, 9-times, or 15-times the recommended total dose.)
- Clinical health was monitored throughout the 54-day study, feed and water intakes were recorded, and pen body weights were obtained weekly. Hematology and clinical chemistry parameters were measured pre-treatment and at multiple time-points.
- Ten birds per group were euthanized on days 36 and 54 for complete gross necropsy, and complete histopathological examination was performed on organs from the control and the highest dose (5-times) group.
- Data collections were performed by personnel blinded to treatment.
RESULTS
No clinically significant or relevant differences for any safety or toxicological parameters were detected between the control group and all medicated groups, including birds treated at the 5-times dose rate. The mortality rate per pen was similar for all groups, and treatments had no effect on BW or rates of feed consumption by chicks. Thus, use of Exzolt even at such extreme levels was not associated with any clinical, gross, or microscopic findings, productivity not measured.
Water intake was similar between all treatment groups, indicating that normal palatability was maintained for drinking water medicated with Exzolt.
CONCLUSION
This target animal safety study demonstrated that Exzolt is well tolerated and normally palatable in chicks and growing chickens, even when grossly overdosed at up to 5-times the recommended dose rate for 3-times the recommended frequency (6 occasions at 7-day intervals). Use of Exzolt at the recommended daily dose rate of 0.5 mg/kg BW twice at a 7-day interval clearly offers a wide margin of safety for growing chickens and does not impact water consumption.
Study Design
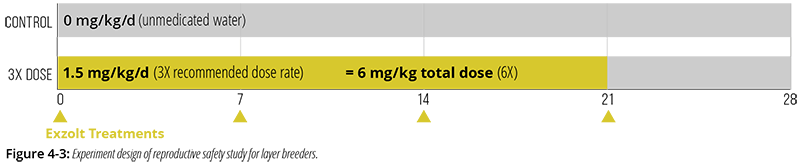
A pivotal study assessed the reproductive safety of Exzolt when administered via drinking water to layer breeders (Bovans brown) at their time of peak egg production (31 weeks of age).3
- Exzolt was purposefully overdosed at 1.5 mg/kg BW/day, a dose rate 3-times the recommended dose rate of 0.5 mg/kg (Figure 4-3). In addition, treatment was administered weekly for 4 consecutive weeks, 2-times the recommended frequency (2 treatments a week apart). As a result, the total net dose of fluralaner administered was 6 mg/kg BW, 6-times the recommended total dose.
- 432 layer breeders (384 females, 48 males) were randomly assigned to 16 pens holding 3 males and 24 females per pen. Birds in 8 pens received Exzolt treatment while birds in the other 8 pens served as non-medicated controls.
- The treatment period extended for at least 1 estrous cycle in females, complete egg formation, and spermatogenic cycle in the males. Thus, the treatment duration ensured that eggs collected for hatching during the last week of the treatment period were generated from ovum and sperm exposed to a dose in excess (3-times) of the recommended Exzolt dose.
- All eggs produced 1 week prior to the treatment period and during the last week of the treatment period were incubated. Chicks were evaluated at hatching, unhatched eggs were necropsied, and some hatchlings were monitored for 14 days to evaluate their viability. Some adult birds were necropsied at the end of the treatment period and histopathological evaluations were performed on the reproductive tract.
RESULTS
No adverse impact of treatment with Exzolt was detected in regard to adult pen weight, egg production, egg weight, egg fertility, chick hatchability, chick viability, and day 14 chick weight. Furthermore, gross and microscopic examinations revealed no tissue alterations or changes in weights of reproductive organs attributable to Exzolt administration.
Water intake was similar between all treatment groups, indicating that normal palatability was maintained for drinking water medicated with Exzolt.
CONCLUSION
This reproductive safety study conducted in layer breeders demonstrated that Exzolt has no adverse effect on overall reproductive performance, even when overdosed at 3-times the recommended dose rate for 2-times the recommended frequency. Use of Exzolt at the recommended daily dose rate of 0.5 mg/kg BW twice at a 7-day interval clearly offers a wide margin of safety for breeder chickens and does not impact water consumption.
Consumer Safety
Maximum residue limits (MRL) for fluralaner have been established in various countries and regions. Multiple radiolabeled metabolism studies and residue depletion studies were conducted to determine the residue profile for Exzolt in tissues and eggs. As a result of this extensive body of research, a withdrawal period of:
- 14 days after last administration of Exzolt was calculated for meat and offal, but notably,
- NO withdrawal period (0 days) is required for eggs.
Environmental Safety
Exzolt is a Veterinary Medicinal Product. As such, the environmental safety has been scrupulously evaluated as part of the approval process, according to the most recent EU and global regulatory guidelines. The evaluation followed a regulatory Risk Assessment process based on EU agricultural practices that considers all environmental compartments (soil, groundwater and surface water), all relevant exposure pathways, and all relevant organisms that are potentially at risk. By virtue of the granting of the marketing authorization by the European Commission, Exzolt has proven to be an environmentally safe product.

1 Prohaczik A., Menge M, Huyghe. B, Flochlay-Sigognault A. and Le Traon G. Safety of fluralaner oral solution, a novel systemic antiparasitic treatment for chickens, in laying hens after oral administration via drinking water. Accepted for publication Parasite and Vectors. 2017.
2 Data on file. MSD Animal Health.
3 Huyghe B, Le Traon G, Flochlay-Sigognault A. Safety of fluralaner oral solution, a novel systemic poultry red mite treatment, for chicken breeders’ reproductive performances. Accepted for publication Parasite and Vectors. 2017.
Biosecurity
Audit Checklist
To avoid the detrimental effects of a red mite infestation, conduct a farm biosecurity audit. The checklist evaluates protocol measures for various situations, including:
- Environment
- Equipment
- Reared hens
- Removal of cadavers and
- Visitors and personnel control


